Arijit Biswas Lab
What do we do?
Understanding the structure of coagulation proteins and their complexes is essential to uncovering their function, particularly in the context of disease mechanisms and translational medicine. While remarkable advances have been made in structural biology, many coagulation proteins remain challenging to characterize due to their unique biochemical and biophysical properties. Despite decades of research into the coagulation cascade, critical structure-function data for many proteins is still lacking—data that is vital for driving therapeutic discoveries and advancements. In our lab, we are dedicated to bridging this gap by exploring coagulation proteins from their genetic origins to their structural and functional roles. By leveraging advanced techniques in structural and computational biology, we study these biomolecules at every level—from individual proteins to complexes, networks, and the proteome. This integrated approach enables us to assess interactions on a system-wide scale, shedding light on the mechanisms of the coagulation cascade and identifying opportunities for disease intervention. Our team possesses extensive expertise in clinical, genetic, epidemiological, structural, and computational biology studies of coagulation factors, with a particular focus on Factor XIII. By combining experimental and computational methods, we aim to uncover the intricate structure-function dynamics of these biomolecules, translating our findings into meaningful insights for both basic science and practical applications in medicine.
Our Focus
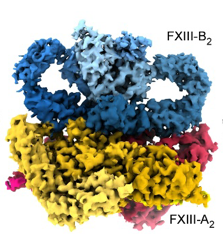
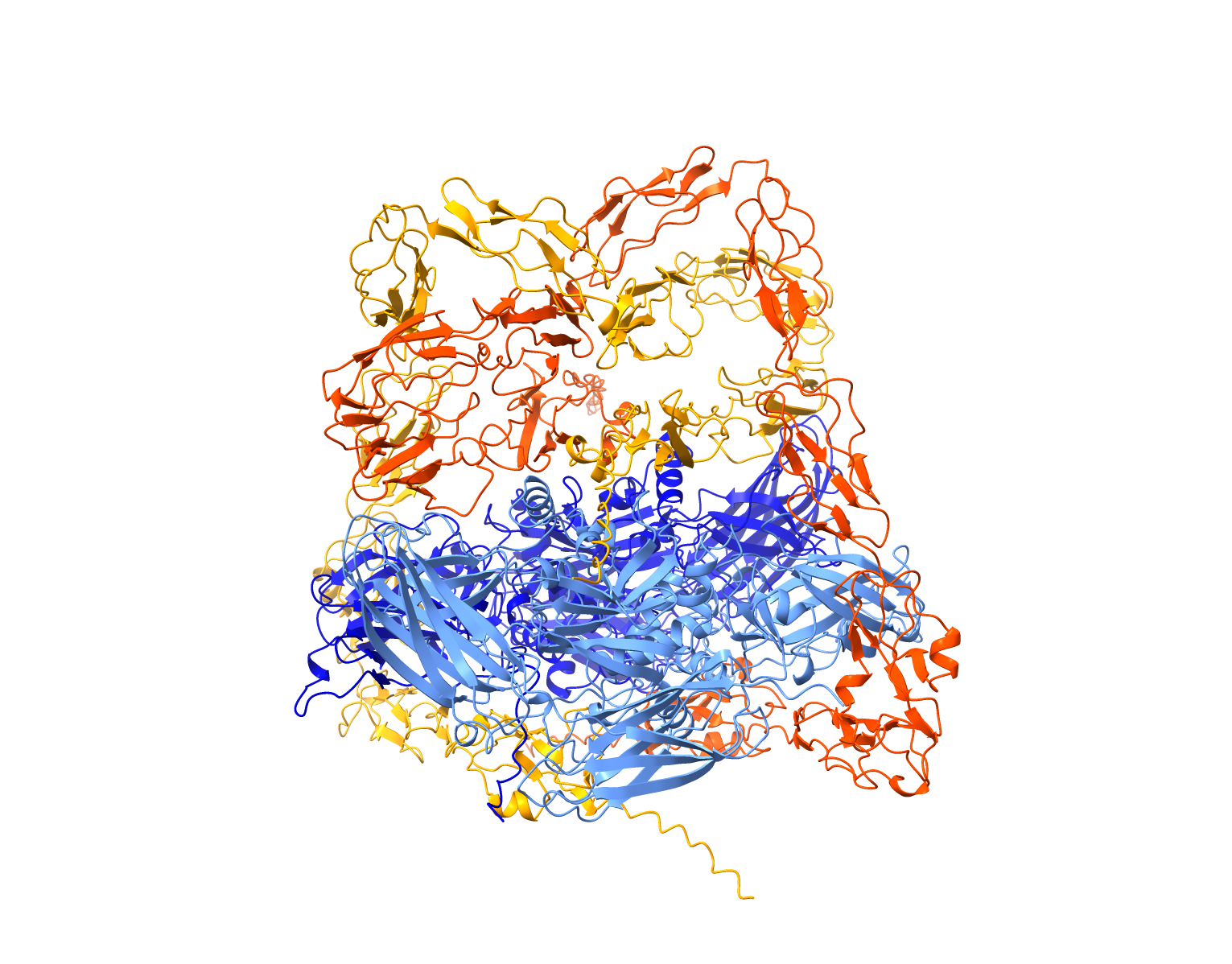
Our group is dedicated to unraveling the genetic, structural, and functional aspects of coagulation proteins through a combination of advanced biochemical and computational approaches. Our primary focus is on Coagulation Factor XIII (FXIII), a plasma-circulating pro-transglutaminase complex responsible for covalently crosslinking soluble fibrin into an insoluble clot, thereby fortifying it against premature fibrinolysis. FXIII deficiency is a rare coagulation disorder with significant clinical implications. The severe form of FXIII deficiency arises from homozygous or compound heterozygous mutations in FXIII genes, resulting in severe bleeding diathesis. Intriguingly, our investigations have suggested the presence of a mild form of FXIII deficiency linked exclusively to heterozygous genetic variants. Using a clustered approach that integrates in-silico modeling with biochemical analyses, we have made significant contributions to understanding FXIII’s activation mechanism, the role of calcium binding, the regulatory importance of the FXIII-B subunit, and the impact of its genetic variability. Our efforts have also led to groundbreaking structural discoveries. Previously, we employed an integrative hybrid approach to construct the first full-atom structural model of the plasma FXIII complex. More recently, we achieved a milestone with cryo-EM analysis, resolving the complete FXIII-A2 subunit at high resolution and partially resolving the FXIII-B2 subunit, offering new insights into its structural organization. In addition to FXIII, our group is also investigating the structural-functional aspects of full-length Coagulation Factor VIII (FVIII). Understanding the structural dynamics of FVIII is essential for addressing its role in the formation of inhibitors, a significant complication in Hemophilia A treatment. By elucidating the molecular mechanisms underlying FVIII’s activity and its implications for disease pathology, we aim to uncover insights that could lead to improved therapeutic strategies for Hemophilia and related conditions. By combining cutting-edge structural and computational biology with biochemical expertise, our group continues to advance the understanding of FXIII, FVIII, and their critical roles in coagulation biology, disease mechanisms, and therapeutic development.
What is coagulation Factor XIII?
- Factor XIII (FXIII) is a human plasma transglutaminase that plays a critical role in the final stage of blood clot formation by stabilizing the pre-formed clot. In plasma, FXIII exists as a heterotetrameric complex, comprising dimeric catalytic FXIII-A subunits non-covalently associated with dimeric regulatory FXIII-B subunits. Upon vascular injury, thrombin-mediated cleavage of the FXIII-A subunits, combined with calcium binding, activates the zymogen, converting it into its active form.
- FXIII deficiency can result in bleeding disorders that range from mild symptoms, such as undetectable nosebleeds, to severe conditions, including fatal intracranial hemorrhages. Additionally, FXIII has been implicated in thrombosis, although its precise role in thrombotic pathophysiology remains under investigation.
- Significant structural insights into FXIII have been achieved. Previously, an all-atom structural model of the plasma FXIII heterotetrameric complex was constructed using an integrative hybrid approach, combining analytical biochemistry, structural biochemistry, and computational modeling. More recently, a cryo-EM structure of the plasma FXIII heterotetrameric complex revealed detailed interaction surfaces between the FXIII-A and FXIII-B subunits, providing new insights into its structural organization.
- Emerging research in thrombosis is now paving the way for the development of FXIII-specific inhibitors as potential tools for thinning clots, offering a promising avenue for future therapeutic advancements.


Our former and present Collaborators and supporters
Our research activities receive support from various sources, including funding from DFG, international research bodies like WFH, and competitive grants from industry partners awarded to PD Dr. Arijit Biswas and Prof. Oldenburg (including Shire, CSL-Behring, Novo Nordisk, Bayer, and Biotest, among others). In addition to our work on FXIII, we collaborate with multiple other groups to investigate the structure and function of coagulation proteins such as vWF, Factor VIII, and VKORC1. We are also engaged in developing inhibitors that can be effective in pro-coagulant scenarios, working closely with Prof. Diana Imhof from the Pharmaceutical Institute at the University of Bonn. We are in a continued collaboration with Geyer Lab, at the BMZ-1 as well. Our strong collaborations improve our capacities tremendously.